All Policies
01. Open Access Policy
All research articles published by the The BioScan are fully open access—freely available to read, download, and share. All the articles are published under the terms of a Creative Commons license, which permits use, distribution, and reproduction in any medium, provided the original work is properly cited.
02. Plagiarism Policy
Author should make clear that manuscripts submitted to the journal for consideration of publication should not be previously publish anywhere either in print or electronic format. Submitted manuscript should not be under consideration by another publication or electronic medium. Copies of related or possibly duplicate materials (i.e. those containing substantially similar content or using the similar data) that have been previously published or are under consideration elsewhere are coming in the plagiarism and such manuscript will be rejected on the first step of publication.
03. COPE Recommendation for Plagiarism
Suspected plagiarism in a submitted manuscript
Suspected plagiarism in a published manuscript
04. Copyright Policy
Copyright of any article submitted to the The BioScan will be retained by the author(s) under the Creative Commons license, which allows unrestricted use, distribution, and reproduction provided the original work is properly cited.
To help researchers who are unable to meet the costs associated with publishing, the The BioScan operates a transparent waiver policy.
Copyright on any open access article published in The BioScan is retained by the author(s). Authors grant THE BIOSCAN a license to publish the article and identify itself as the original publisher. Authors also grant any third party the right to use the article freely as long as its integrity is maintained and its original authors, citation details and publisher are identified.
The entire article published by The BioScan is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License which permits commercial and non-commercial re-use of an open access article, as long as the original author and source are properly attributed.
05. Rights and Grants to the Author
The author has to sign the licence agreement to allow the author to publish the article. A license agreement is related to the transfer of publishing rights from author to publisher.
The copyright of the submitted article will be retained by the author.
The author will also retain patent, trademark, and other intellectual property rights for his submission.
06. Licensing Policy
All articles published by the TheBioScan are licensed under an open access Creative Commons Attribution 4.0 International License, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use, re-use and exposure of the work, while ensuring that the authors receive proper credit.
Therefore, all authors must agree on submission to have their published article available under the Creative Commons Attribution 4.0 International License.
07. COPE Recommendation for Conflict of Interest
Undisclosed conflict of interest (Col) in a submitted manuscript
Undisclosed conflict of interest (CoI) in a published article
Permission is required for:
1. Your own works published by other Publishers and for which you did not retain copyright.
2. Substantial extracts from anyones' works or a series of works.
3. Photographs for which you do not hold copyright.
Permission is not required for:
1. Reconstruction of your own table with data already published elsewhere. Please notice that in this case you must cite the source of the data in the form of either "Data from..." or "Adapted from...".
2. Reasonably short quotes are considered fair use and therefore do not require permission.
3. Graphs, Charts, Schemes and Artworks that are completely redrawn by the authors and significantly changed beyond recognition do not require permission.
08. Publication Ethics and Malpractice Statement
The BioScan is dedicated to upholding the ethical behavior and standards of publication throughout the publication process.
Key expectations of editors, peer-reviewers, and authors are summarized below:
Editors
Duties should be carried out by editor in fair way without discrimination on any basis. Chief editor is responsible for final decision on publication of manuscript.
Decision on manuscript shall be solely on the intellectual and academic merit of manuscript without commercial influence.
Editors and editorial staff must not disclose any information or content of the manuscript to anyone except authors, reviewers and editorial staff of the concerned manuscript.
In the event of complaint or conflict, a fair and reasonable procedure must be followed according to the policies and procedure of journal. Authors must be required to address and respond to the complaint or conflict. Communication or document related to grievance or conflict should be retained by chief editor. Submitted manuscript must be screened for plagiarism and plagiarised manuscript must be immediately rejected without further review.
Unpublished or rejected manuscript material disclosed in submitted manuscript confidentiality must be maintained.
Authors
Author must ensure that submitted manuscript is completely original and if authors have adopted or used any work of others this must be properly cited or acknowledged or quoted. Author must obtain permission to reproduce any content or figure or picture from other sources.
Author must not submit the same manuscript to multiple journals at the time because such action constitutes unethical publishing practice and is unacceptable. Manuscripts violating ethical practice of publication will be immediately rejected without further review.
Author must declare potential conflicts of interest which might be financial or other applicable conflicts of interest and could be influencing the duties of author, results or interpretation of their manuscript. Sources of support must be disclosed in manuscript.
Author must cite the ethical statement in the studies involving human or animals and must confirm that approval has been taken prior to the study. These studies must conform to institutional, local, national and international laws and requirements. In case of human subjects author must cite consent of subjects and must not disclose personal data or their identification in any form.
Author should inform to the chief editor if any substantial corrections or errors are found in their publication so that error can be corrected as an erratum, addendum or retraction of manuscript whichever necessary and applicable.
Authorship in manuscript should be limited to those who have made substantial contribution to the manuscript. Other non-significant contributors should be acknowledged wherever necessary. Corresponding author must be responsible for ensuring that all co-authors have agreed to be the part of submitted manuscript and have approved the final version of manuscript before publication.
Reviewers
Reviewers must maintain the confidentially of the manuscript and must not show or discuss the manuscript with others except chief editor.
Reviewers must alert to the chief editor in case they find that manuscript under review has similarity with published material.
Reviewers must alert the chief editor in case they find potential conflict of interest with authors of manuscript or anyone associated as contributor in manuscript. If deemed necessary, reviewer must not review manuscript involving potential conflict of interest.
Reviewers must alert the chief editor if any sources are not cited or acknowledged in manuscript.
09. APC Waiver Policy
All articles published by The BioScan are fully open access and not require Article Processing Charges.
To help researchers who are unable to meet the costs associated with publishing, The BioScan operates a transparent waiver policy.
10. Withdrawal Policy
Some authors request withdrawal of manuscript from the publication process after submission. In some instances the request for withdrawal is made within a few days after submission, however, many times the request is made very late, when the manuscript is only a few days away from publication in the journal. Withdrawing manuscripts from publication wastes the valuable resources and tremendous amount of effort made in processing the manuscripts by the editors, reviewers and the editorial staff.
To avoid withdrawal of a manuscript we sincerely request the authors especially the corresponding author, first author and guarantor of submission, to address the following issues before submitting the manuscript for The BioScan
Only email communication regarding withdrawal of manuscript will be considered. Withdrawal request should be sent to editorial.thebioscan@gmail.com
11. Human and Animal Rights Policy
All research studies on humans (individuals, samples, or data) must have been carried out following the principles of the WMA Declaration of Helsinki. In the case of human participants, or any data related to their biological material, authors should provide an appropriate institutional and/or national research ethics committee statement confirming that the study was approved (or granted exemption) by the committee. Furthermore, the author should certify that the study was performed in agreement with the ethical standards provided in the WMA Declaration of Helsinki (1964) (WMA Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects – WMA – The World Medical Association) and its latest amendments or comparable ethical standards. If any doubt exists as to whether the research was performed following the 1964 Helsinki Declaration or equivalent standards (WMA Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects – WMA – The World Medical Association), the authors must elaborate the rationale for their approach and indicate that an independent institutional ethics committee/review board explicitly approved the ambiguous components of the research work. In case the work was provided with relaxation from obtaining ethics approval, the same should also be highlighted in the manuscript, citing the grounds on which relaxation was accorded.
12. Errata Retractions and Corrigenda Policy
Erratum
An erratum refers to a correction of errors introduced to the article by the journal in editing or production.
All Journal introduced changes are highlighted to the author at the proof stage and any errors are ideally identified by the author and corrected by the publisher before final publication.
Corrigendum
Corrigenda submitted by the original authors are published if the scientific accuracy or reproducibility of the original paper is compromised. A corrigendum refers to a change to their article that the author wishes to publish at any time after acceptance. Authors should contact the editor of the journal, who will determine the impact of the change and decide on the appropriate course of action.
The process for handling cases requiring corrections, retractions, and editorial expressions of concern
In The BioScan , we make sure that our published journals adhere to the guidelines set forth by the International Committee of Medical Journal Editors (ICMJE) for the conduct, reporting, editing, and publication of scholarly work in medical journals. Additionally, we follow the guidelines established by the Committee on Publication Ethics (COPE)
The goal of THE BIOSCAN is to maintain the accuracy of academic publications. If an error or misleading information is discovered, it must be corrected promptly and prominently. Should an investigation reveal fraudulent activity, the publication in question will be retracted and clearly marked for readers and indexing systems.
Corrections
Sometimes mistakes are found in published papers and the Editor-in-Chief may choose to inform the readership of the error by publishing a corrigendum or erratum. This will be a new article in the journal that corrects the mistake and references the original article.
Retractions
Retractions may be published if an article has significant errors that weaken the conclusions or if unethical practices such as plagiarism or duplicate publication are found.
At THE BIOSCAN, we follow industry best practices and adhere to COPE guidelines when confirming a retraction:
- When an article needs to be retracted, a note titled "Retraction: [article title]" is published in a later issue of the journal and included in the contents list. The note is signed by either the authors or the editor.
- The electronic version includes a link to the original article.
- Before accessing the online article, readers will first encounter a screen with a retraction note. This is where the provided link will lead them, and from there they may proceed to the article itself.
- The original article remains the same, except for the addition of a watermark on the HTML and PDF indicating that it has been retracted on each page.
13. Digital Preservation
The The BioScan is fully open-access, with articles freely available on the journal's website, including PDF. All published articles of our journal are permanently archived by CLOCKSS. Authors are also encouraged to self-archive the published articles in their own or institutional websites as well as governmental websites, library websites, without permissions required.
Data deposit:
Authors are expected to deposit any data and related metadata arising from the research in an appropriate public repository (for example, GenBank for gene sequences), if they are not already provided as part of the submitted manuscript or in its supplementary materials.
14. Advertisement Policies
The The BioScan accepts advertisements for online publication on its website. The Journal will adhere to all ethical and commonly accepted advertising practices and will ensure that its practices conform to association policy. The Journal reserves the right to reject any advertisement deemed not relevant or consistent with the above or to the aims and policies of THE BIOSCAN. The BIOSCAN has the right to refuse any advertisement that is inappropriate or incompatible with our mission and to stop accepting any advertisement previously accepted.
All the selected advertisements are subject to editorial approval. All orders must be paid in full by the stated deadlines. The appearance of advertisements on these web sites does not imply endorsement of the advertised company or product, nor is advertising allowed to affect editorial decisions or editorial content. Contracts and individual insertions may be cancelled up through the closing date for the issue; ads cancelled after this date will be billed at the regular space rate.
Contact- editorial.thebioscan@gmail.com for more details.
TARIFFS FOR ADVERTISEMENT
USD 700 (INR 20000/-)
For advertisement on home page along with all the other pages of THE BIOSCAN website (For one year)
USD 500 (INR 10000/-)
For advertisement on other pages than home page of THE BIOSCAN website (For one year)
THE BIOSCAN will not accept advertisements related to alcohol, tobacco, weapons, firearms, ammunition, fireworks, gambling, lottery, pornography, political or religious content, claims to offer a “miracle” cure or method, and make unsubstantiated health claims on its web site.
Also, THE BIOSCAN will not accept advertisements that are designed to collect personally identifiable information from visitors to the THE BIOSCAN Web site without their knowledge or permission.
Correspondence
Managing Editor
THE BIOSCAN
[editorial.thebioscan@gmail.com]
15. Editorial Policies
The BioScan provides immediate, free, and open access to its content on the principle that making research freely available to the public supports a greater global exchange of knowledge. The major emphasis is laid on the inter-disciplinary nature of the work. The main criteria for acceptance of the articles are novelty, clarity, and significance as relevant to a better understanding of pharmacy, experimental biology, and agricultural & medical sciences. The entire journal published by HPI complies with the International Committee of Medical Journal Editors’ uniform requirements for manuscripts.
16. Conflict of Interest and financial disclosures
Conflicts may be financial, academic, commercial, political or personal. Financial interests may include employment; research funding (received or pending), stock or share ownership, patents, payment for lectures or travel, consultancies, non financial support, or any fiduciary interest in a company. When any author(s) has such type of financial or personal obligation, it is required to mention such potential conflict of interest along with financial interests and related affiliations (other than those affiliations listed in the title page of the manuscript) relevant to the subject of their manuscript. For all accepted manuscripts, author must declare such conflicts of interest at the end of the manuscript before the manuscript publish. Author without a conflict of interest should include a statement that no such conflicts exist.
COPE Recommendation for Conflict of Interest
- Undisclosed conflict of interest (Col) in a submitted manuscript
- Undisclosed conflict of interest (CoI) in a published article
Funding / Support and Role of Sponsor
All the author(s) who have received financial assistance for carried out research work and publication should be described clearly in an Acknowledgment section of the manuscript.
Data access and responsibility
Regardless of funding source for any manuscript containing original data, corresponding author must indicate in acknowledgement section that he/ she “had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis”. Modified statements or generic statements indicating that all authors had such access are not acceptable.
Acknowledgement section
The acknowledge section should be at the end of the manuscript text but before the reference section. It should includes the list of contributor(s), information on author access to data, conflicts of interest including financial interests and relationships, sources of funding and support like information’s. Authors should obtain written permission from all individuals whose names included in the Acknowledgment section and the corresponding author must confirm that such permission has been obtained in the Authorship Form.
Ethical approval of studies
For all manuscripts reporting data from studies involving human participants or animals, formal review and approval, or formal review and waiver, by an appropriate institutional review board or ethics committee is required. For investigations involving humans, state in the Methods section whatever oral or written informed consent was obtained from the study participants. Editors may request that authors provide documentation of the formal review and recommendation from the institutional review board or ethics committee responsible for oversight of the study.
COPE Recommendation for Ethical issues
Recommendation against suspicious ethical problem with a submitted manuscript
17. Participant/Patient privacy and informed consent
All individuals have their basic rights which cannot and must not be violated. The author is solely responsible for taking care of the same. Individuals taking part in the study have absolute right and authority to decide what happens to the (identifiable) personal data collected through their interview or other means including photography. Particular attention is to be paid concerning the images of assailable people such as minors, patients, or refugees or the use of images from a sensitive perspective. In case any respondents intend to secure their information, the author should refrain from publishing personal identifying details like names, dates of birth, biometrical characteristics, or other distinguishing attributes of the participants that were studied unless the information is warranted for scholarly purposes and the respondents have given explicit written informed consent for the publication. Authors are required to obtain informed consent if there is any doubt. For instance, merely masking the eye region in photographs of participants is not adequate protection against obscurity. If identifying attributes are modified to protect anonymity, for instance in genetic data, the authors must substantiate that the changes done have not altered the intended meaning. In the case of medical/diagnostic images such as X-rays, brain scans, ultrasound, laparoscopic, and pathology slides images, consent from the participant is not necessary. However, if there is a possibility of distinctive information with images, authors are required to obtain the consent of participants. If published images are being reused from prior publications, authors must obtain the permission of the original source. Without prejudice, the publisher assumes that prior publication obtained the necessary and relevant information regarding the consent of participants. However, authors should provide the appropriate attribution in case of the images taken from the published sources.
In the case of children, adolescents, or incapacitated study participants written informed consent must be obtained from their respective parents or guardian. In a situation where verbal informed consent was obtained instead of written informed consent, this must be highlighted within the manuscript along with the appropriate reasons.
As per the principles outlined in the Nuremberg Code and the Belmont Report, informed consent must have been given with a free will, without any force or influence, and under no coercion or bribery. The editorial office also scrutinized the submitted manuscript to protect the vulnerable group and upon request, the editorial office will provide supported documentary evidence (blank consent forms and any related discussion documents from the ethics board). In addition, in the studies which highlight categories by race, disability, gender, ethnicity, disease, etc., a suitable explanation regarding why such categorization was needed must be clearly stated in the article.
The protection of vulnerable groups and individuals
Clinical trials
Apart from the informed written consent as needed (as stated above), Authors should ensure that the protocols for clinical trials are registered in a publicly accessible registry before the recruitment of participants. The public registry must be accessible to all prospective registrants and handled by a non-profit entity. For reference, a list of eligible registries can be seen at the WHO International Clinical Trials Registry Platform (ICTRP). Moreover, such trials may also be enrolled at Clinical Trials.gov or the EU Clinical Trials Register and the trial registration number and registration date must be included in the Abstract and Methods section of the manuscript. In addition, any departure from the initial trial protocol must be highlighted along with its rationale.
Mechanisms for ensuring the quality of published articles
To ensuring the scientific quality of published articles, all the submitted articles are scrupulously peer reviewed by high quality researchers, editorial and advisory team to accept the high quality submissions and the quality is controlled by the Editor(s)-in-Chief or subject editors. All the members of editorial are experts in their field from various countries and regions around the world. These members are well qualified and have high level publication experience expert. The published articles reflect the up-to-date research findings, with reliable results, objective and un-biased discussion of the results. The highest quality standards are achieved by following the guide line of DOAJ Transparency & best practice and COPE CORE PRACTISE
18. Informed consent policy
Obtaining informed consent before medical treatment is crucial in both ethical and legal terms. THE BIOSCAN ensure the process of informed consent adheres to the U.S. Department of Health and Human Services (HHS) NCBI-NLM guidelines, as outlined in Shah et al. 2022
19. Data Sharing Policy
The The BioScan adheres to the Data Management and Sharing (DMS) policy issued by the National Institutes of Health (Data Management and Sharing (DMS) policy). This policy ensures that scientific data accompanying published articles are shared in an equitable manner with users, thus promoting the acceleration of biomedical research discovery. The validation of research results, accessibility to high-value datasets, and promotion of data reuse for future research studies are all facilitated by the sharing of published research article data. THE BIOSCAN strictly follows the NIH Policy for Data Management & Sharing to share the published article data. THE BIOSCAN strictly follows the guidelines of the National Institutes of Health (NIH) Policy for Data Management and Sharing . Our commitment to this policy ensures that any data associated with our published articles will be made available for sharing and dissemination in accordance with the NIH's regulations.
Principles of Transparency and Best Practice in Scholarly Publishing
All the journal published by HPI strictly following the guide line of PRINCIPLES OF TRANSPARENCY AND BEST PRACTICE IN SCHOLARLY PUBLISHING describe by Directory of Open Access Journal available on https://doaj.org/bestpractice.
20. Submission of Manuscript
- Submission of a manuscript to the THE BIOSCAN implies that the research work described has neither been published, nor it is under consideration for publication anywhere else and the manuscript has been approved by all co-authors.
- It is the responsibility of author (s) to obtain prior approval of the appropriate authorities of the institute where the work had been carried out. The publisher in no case, whatsoever, will be held legally responsible if there were any claims raised for compensation.
- If authors are using any copyrighted material (figure, table or text passages), they must obtain permission from the copyright owner(s) for doing that. Authors are fully responsible for the authenticity of the literature and originality of the data. The THE BIOSCAN will not be responsible for the plagiarism.
- We receive nominal fee from the authors as the manuscript handling charges after acceptance of their manuscript by our reviewer panel. The amount of fee payable is dependent upon several factors including author's affiliation, manuscript length etc. For fee detail, before submission manuscript author must visit https://thebioscan.com/index.php/pub/issue/archive However, publication of article in our journal is not contingent upon author's capability to remit the fee. Partial waiver in fee is also granted to the deserving and needy authors. Priority in waiver is accorded to the budding researchers.
- Before submitting an article to the The BioScan, authors should visit NLM's important policies to improve the quality of their work. Authors can enhance the quality of their articles by following these policies.
- Human research: Helsinki Declarationas revised in 2013
- Systematic reviews and meta-analyses: PRISMAguidelines (for protocols, see the PRISMA-P guidelines)
- Case reports: the CARE case report guidelines
- Clinical trials: CONSORT(for protocols, see the SPIRIT guidance)
Animal studies: ARRIVE and Guide for the Care and Use of Laboratory Animals
21. Manuscript Preparation
-
Manuscript prepared following the guidelines strictly substantially reduces the time taken in editorial processing and results into rapid decision.
- Manuscript should be submitted electronically as single word file embedding all the figures and tables in following format:
- Font: Times New Roman; Font size- 14 for article title, 13 for section titles and 12 for text.
- Line Space: Manuscript must be typed in Double line space.
- Page setup: 1” margin in all sides.
- English: The THE BIOSCAN is published in English and all manuscripts should be written in English. A poor level of English may slow, or even prevent, the publication of the manuscript. The THE BIOSCAN recommends that authors who are not native English speakers get their manuscripts edited for the English language. Authors willing to avail paid services of professional language editors may contact editorial.thebioscan@gmail.com. However, this referral is just to facilitate authors to improve the language of their manuscript. It is neither mandatory to submit the manuscript using this service, nor does it guarantee of acceptance of manuscript for publication in THE BIOSCAN. The decision of editorial board shall be final in this regard.
- The manuscript should be structured as word file in following pattern:
Title page: It should include article title, name of author (s), affiliations, full contact address of corresponding author with Phone, Fax and Email.
Next page will contain abstract of the article (150-250 words) followed by 5-6 keywords. The manuscript text will start from the subsequent page.
Body: The manuscript body will consist of
- Introduction: This section will give brief introduction of the research work while reviewing the latest research related to it. The introduction section should clearly mention the significance and objectives of the research work being reported.
- Materials & Methods: This section should be detailed enough so that the other interested researchers could repeat the experiments. In case of common methods used, only citation will be sufficient. However, if any modification in the established method was made, it should be clearly mentioned. Wherever applicable, it should also be mentioned that the ethical clearance was obtained for performing animal trials.
- Results and Discussion: Authors should describe their important observations in this section. The data should have been analysed stastically
- Conclusions: In this section, authors should discuss their observations in view of already published literature. Variation from the published reports, if any, should be discussed logically giving all possible reasons and then conclusions should be made.
- After “Discussion and conclusions” section, authors can acknowledgethe technical help/financial grant of their colleague/institutes etc.
Conflicts of interest and financial disclosures: Conflicts may be financial, academic, commercial, political or personal. Financial interests may include employment; research funding (received or pending), stock or share ownership, patents, payment for lectures or travel, consultancies, non financial support, or any fiduciary interest in a company. When any author(s) has such type of financial or personal obligation, it is required to mention such potential conflict of interest along with financial interests and related affiliations (other than those affiliations listed in the title page of the manuscript) relevant to the subject of their manuscript. For all accepted manuscripts, author must declare such conflicts of interest at the end of the manuscript before the manuscript publish. Author without a conflict of interest should include a statement that no such conflicts exist.
Please note that The BioScan follows the following Reference Style, Citation Style Name Year and Numbering Style Chapter Content.
In text of manuscript
Morse 2009 (Single Author) ; Morse and Buhler 1997; (Double Author) ; Jiménez et al. 2000 (Multiple author); Barrera 2006, 2020/ Morse and Buhler 1997, 2019/ Jiménez et al. 2000, 2005, 2010 (Multiple work of a single/double/ multiple author); (Savory and Butterfield 1999; Savory Global 2019) – Multiple citations in favor of single fact
In reference section
For Book
- Altieri, M. A. (1987). Agroecology: The scientific basis of alternative agriculture. Boulder: Westview Press.
- Mitchell, J.A., Thomson, M., & Coyne, R.P. (2017). A guide to citation. London, England: My Publisher.
For Journal Articles
- Altieri, M. A., & Masera, O. (1993). Sustainable rural development in Latin America: Building from the bottom-up. Ecological Economics, 7(2), 93–121.
- Gashaw, B. A., Habteyesus, D. G., & Nedjo, Z. S. (2018). Determinants of coffee value addition by smallholder farmers in Jimma Zone, Ethiopia. The International Journal of Business Management and Technology, 2(4), 112–123.
- Avelino, J., Cristancho, M., Habteyesus, D. G., Georgiou, S., et al. (2015). The coffee rust crises in Colombia and Central America (2008–2013): Impacts, plausible causes and proposed solutions. Food Security, 7, 303–321 (If more than six authors).
Edited Book
- Williams, S.T. (Ed.). (2015). Referencing: A guide to citation rules (3rd ed.). New York, NY: My Publisher.
Edited Book Chapter
- Altieri, M. A., & Nicholls, C. I. (2003). Ecologically based pest management: A key pathway to achieving agroecosystem health. In D. J. Rappoport, W. L. Lasley, D. E. Rolston, et al. (Eds.), Managing for healthy ecosystems (pp. 999–1010). Boca Raton: Lewis Publishers.
E-Book
- Mitchell, J.A., Thomson, M., & Coyne, R.P. (2017). A guide to citation. Retrieved from https://www.mendeley.com/reference-management/reference-manager
E-Book Chapter
- Troy, B.N. (2015). APA citation rules. In S.T, Williams (Ed.). A guide to citation rules (2nd ed., pp. 50-95). Retrieved from https://www.mendeley.com/reference-management/reference-manager
Online Content
- Troy, B.N. (2015). APA citation rules. Retrieved from https://www.mendeley.com/reference-management/reference-manager
FAO Guideline (2020) Food-based guidelines for dietary submission Retrieved from https://www.mendeley.com/reference-management/reference-manager
22. Check List
As part of the submission process, authors are required to check off their submission's compliance with all of the above said content, and submissions may be returned to authors that do not adhere to author guidelines.
Sample Papers
Author can download sample Papers from https://thebioscan.com/index.php/pub/issue/archive
23. Article Processing Charge
We do not charge anything for publication or subscription. The BioScan Journal is an open-access journal that can be subscribed to free of charge. There are no fees for publication, editing, or processing.
24. Author’s Agreement form
Author (s) will submit an THE BIOSCAN Author Agreement Form in the specified format for copyright transfer and declare about authenticity of the literature and originality of the data.
25. English Language Editing
Many researchers who are not native English speakers often find it hard to get published due to their manuscripts being rejected as the English is not good enough. The poor English stops much valuable research work from being published; the data and results are more than suitable for publication, but they are hidden behind the inadequate English. Authors are need to write the manuscript in English (US) with proper care of sentences construction and any grammatical error/s which make it suitable for review and publication by THE BIOSCAN.
26. Peer Review Process
All articles submitted to the The BioScan are subjected to a rigorous completeness check before undergoing evaluation by a subject editor. Following this, the manuscript is reviewed by two external reviewers who are considered experts in the relevant content. While the editor takes into account the peer-reviewed reports, they are not obligated to follow the recommendations provided by the reviewers when making a decision. If concerns are raised by a single reviewer or the editor, the manuscript may be rejected. The authors will receive the editorial decision alongside the peer review reports.
The selection of reviewers for peer review is an essential aspect of the publication process, and numerous factors are taken into consideration. These include the reviewer's expertise, reputation, recommendations, potential conflicts of interest, and past performance. We prioritize reviewers who exhibit quickness, thoroughness, logical reasoning, and a cooperative attitude. Demonstrating excellence in these areas is highly valued in our peer review process.
The BioScan employs a rigorous double-blind peer-review system that ensures complete anonymity between authors and reviewers. HPI places great emphasis on delivering fair and unbiased evaluations, without favoring either the author or reviewer. The peer-review procedure is executed in accordance with strict guidelines.

The BioScan strictly follows double blind peer-review system by maintaining complete anonymity between author and reviewers. The BioScan prefers fair judgment without any bias to author or reviewer. The detail of peer review procedure is as below.
Credits
This peer review policy and procedure is based on https://www.elsevier.com/en-gb/reviewers/what-is-peer-review and https://grants.nih.gov/policy/peer/index.htm#new
27. Editorial policies
Certainly! Editorial policies are essential guidelines that underpin the commitment of leading research publishers to editorial independence and supporting research excellence. Let’s explore some key aspects of editorial policies:
Artificial Intelligence (AI):
We believe in using AI responsibly for the benefit of the research community, authors, editors, readers, and staff.
Our approach involves adopting an ethically-focused stance while designing, developing, and deploying AI solutions.
We prioritize human-centered values in the responsible use of AI, as reflected in our AI Principles and editorial policies.
Notably, we do not attribute authorship to AI, exclude generative AI images from our publications, and ask peer reviewers not to upload manuscripts into generative AI tools1.
Author Contributions:
We emphasize clear crediting of every author’s contribution to a research paper.
Authors are required to specify their individual contributions in the manuscript.
Collaboration with scholars and relevant stakeholders in research locations is encouraged, with inclusion as co-authors when they meet authorship criteria1.
Respectful Communication:
We build trust through relationships based on mutual respect.
Professional and respectful behavior is expected when engaging with authors, reviewers, and readers.
Aggressive behavior, harassment, bullying, or discrimination against our staff is not tolerated1.
Disclosure of Competing Interests:
Authors, peer reviewers, and editors must disclose any competing interests that could influence decisions and conclusions.
Declarations of competing interests are required in selected journals and fields, with plans to implement them across our entire portfolio.
Remember, these policies uphold the integrity of scholarly publishing and contribute to a robust research ecosystem.
28. Publisher Policies
Certainly! Publisher policies play a crucial role in maintaining the quality and integrity of content within the advertising ecosystem. Let’s delve into the details:
Content Policies:
Illegal Content: We strictly prohibit content that is illegal, promotes illegal activities, or infringes on the legal rights of others.
Intellectual Property Abuse: Content infringing copyright or promoting the sale of counterfeit products is not allowed.
Dangerous or Derogatory Content: We do not permit content that incites hatred, promotes discrimination, or disparages individuals or groups based on various characteristics1.
Behavioral Policies:
These policies govern user behavior and interactions with ads. For instance, we discourage deceptive practices, click fraud, and manipulative tactics.
We aim to maintain a positive user experience by ensuring ads are relevant and non-intrusive.
Video Policies:
Video content must adhere to guidelines related to violence, sexual content, and other sensitive topics.
We prioritize user safety and appropriate content in video ads.
Privacy-Related Policies:
We respect user privacy. Publishers must handle personal data responsibly and comply with privacy laws.
Advertisers should provide clear information about data collection and usage.
Requirements and Other Standards:
Publishers must meet certain technical requirements to ensure smooth ad delivery.
Ad placements should align with the overall user experience on the website or app.
Remember, these policies are essential for maintaining trust in the advertising ecosystem and ensuring a safe and relevant experience for users and advertisers alike. If you’re a publisher using Google ad code, adhering to these policies is crucial to avoid potential account suspensions or ad blocks.
29. REVIEWERS GUIDELINES
- All the reviewers are requested that before accepting to review a manuscript they should ensure the following:
- The manuscript is within their area of expertise.
- They can dedicate the appropriate time to conduct a critical review of the manuscript.
- All the reviewers should declare their conflict of interest and can decline the review if conflicts exist.
- The BioScan follows the blind review process so the manuscript and the review process should remain confidential during and after the review process. Reviewers should ensure it on their part.
- Review of a manuscript should be fair so reviews should be honest and should not influenced by:- The origin of the manuscript
- Religious, political or cultural viewpoint of the author
- Gender, race, ethnicity or citizenry of the author
- In evaluating a manuscript, reviewers should focus on the criteria decided by The BioScan (Click here for manuscript evaluation form)
- Reviewers should only accept manuscript that they are confident that they can dedicate appropriate time in reviewing. Thus, reviewers should review and return manuscripts in a timely manner.
30. Editorial Process
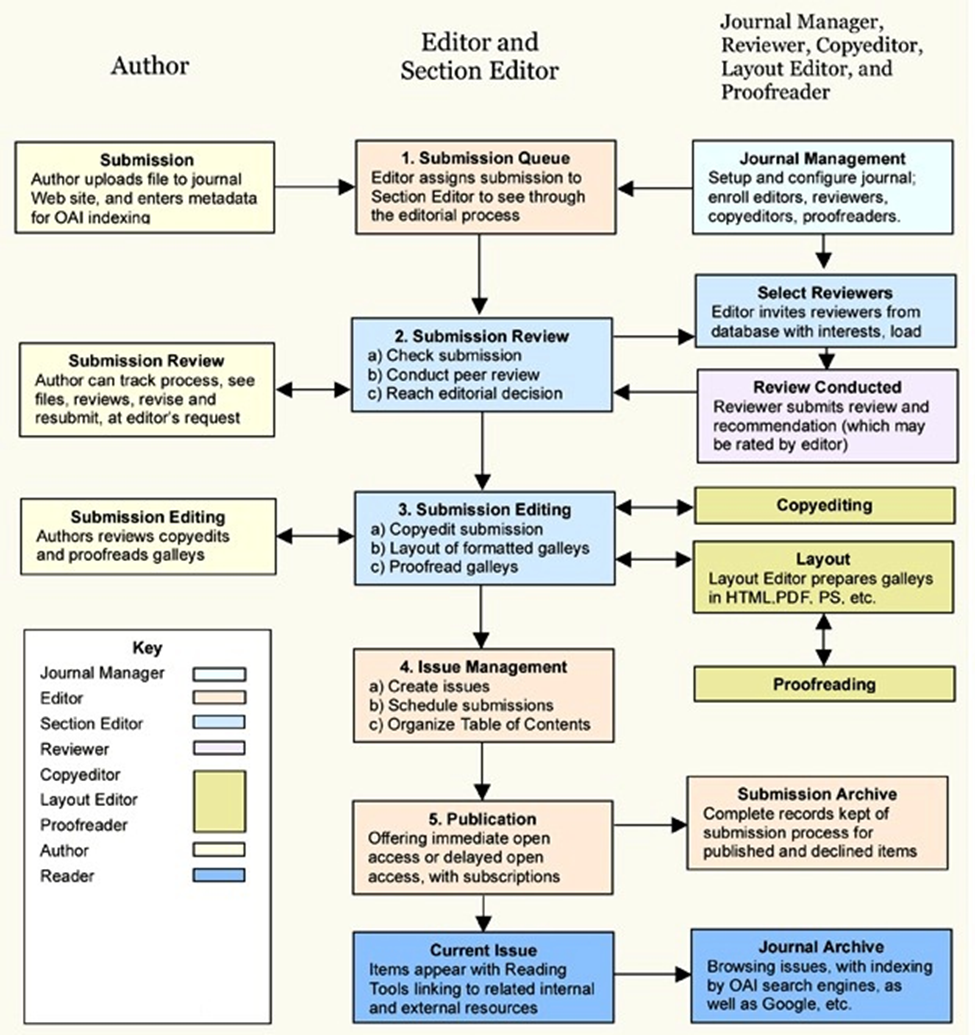
Credits
This publishing system is based on source code obtained from the Public Knowledge Projects Open Journal System.
The Public Knowledge Project is dedicated to improving the scholarly and public quality of research. It operates through a partnership among the Faculty of Education at the University of British Columbia, the Simon Fraser University Library, the School of Education at Stanford University, and the Canadian Centre for Studies in Publishing at Simon Fraser University.

























